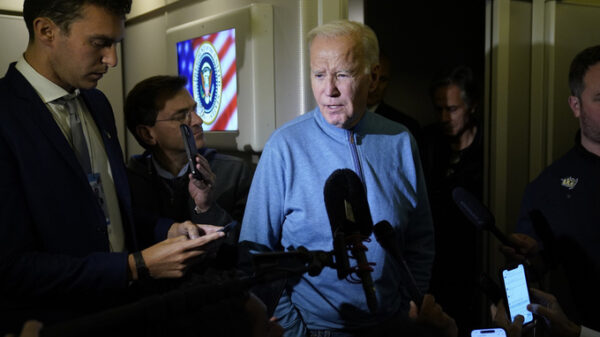
FILE – In this March 2, 2021, file photo, pharmacy technician Hollie Maloney loads a syringe with Pfizer’s COVID-19 vaccine at the Portland Expo in Portland, Maine. The U.S. gave full approval to Pfizer’s COVID-19 vaccine on Monday, Aug. 23, 2021. (AP Photo/Robert F. Bukaty, File)
WASHINGTON (AP) — The U.S. gave full approval to Pfizer’s COVID-19 vaccine on Monday, a milestone that may help lift public confidence in the shots as the nation battles the most contagious coronavirus mutant yet.
The vaccine made by Pfizer and its partner BioNTech now carries the strongest endorsement from the Food and Drug Administration, which has never before had so much evidence to judge a shot’s safety. More than 200 million Pfizer doses already have been administered in the U.S. — and hundreds of millions more worldwide — since emergency use began in December.
“The public can be very confident that this vaccine meets the high standards for safety, effectiveness and manufacturing quality the FDA requires of an approved product,” said acting FDA Commissioner Janet Woodcock. “Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.”
The U.S. becomes the first country to fully approve the shot, according to Pfizer, and CEO Albert Bourla said in a statement he hoped the decision “will help increase confidence in our vaccine, as vaccination remains the best tool we have to help protect lives.”
U.S. vaccinations bottomed out in July. As delta fills hospital beds, shots are on the rise again — with a million a day given Thursday, Friday and Saturday. Just over half of the U.S. population is fully vaccinated with one of the country’s three options, from Pfizer, Moderna or Johnson & Johnson.
The FDA’s action also may spur more vaccine mandates by companies, universities and local governments. This month New York City, New Orleans and San Francisco all imposed proof-of-vaccination requirements at restaurants, bars and other indoor venues. At the federal level, President Joe Biden is requiring government workers to sign forms attesting that they’ve been vaccinated or else submit to regular testing and other requirements.
Anxious Americans increasingly are on board: Close to 6 in 10 favor requiring people to be fully vaccinated to travel on airplanes or attend crowded public events, according to a recent poll by The Associated Press-NORC Center for Public Affairs Research.
“Mandating becomes much easier when you have full approval,” said Dr. Carlos del Rio of Emory University. “I think a lot of businesses have been waiting for it.”
The FDA, like regulators in Europe and much of the world, initially allowed emergency use of Pfizer’s vaccine based on a study that tracked 44,000 people 16 and older for at least two months — the time period when serious side effects typically arise.
That’s shorter than the six months of safety data normally required for full approval. So Pfizer kept that study going, and the FDA also examined real-world safety evidence in deciding to fully license the vaccine for people 16 and older, those studied the longest. Pfizer’s shot still has emergency authorization for 12- to 15-year-olds.
Even after hundreds of millions of shots, serious side effects — such as chest pain and heart inflammation in teens and young adults — remain exceedingly rare, the FDA said.
As for effectiveness, six-month tracking of Pfizer’s original study showed the vaccine remained 97% protective against severe COVID-19. Protection against milder infection waned slightly, from a peak of 96% two months after the second dose to 84% by six months.
Those data came before the extra-contagious delta variant began spreading, but other data from the Centers for Disease Control and Prevention shows the vaccine is still doing a good job preventing severe disease caused by that mutant.
As for all the talk about booster doses, the FDA’s licensure doesn’t cover those. The agency will decide that separately.
The FDA already is allowing emergency use of a third dose of either the Pfizer or Moderna vaccine for people with severely weakened immune systems, such as organ transplant recipients who don’t respond as strongly to the usual two shots. For everyone else who got those vaccinations, the Biden administration is planning ahead for booster starting in the fall — if the FDA and CDC agree.
Also still to be decided is vaccination of children under 12. Both Pfizer and Moderna are studying youngsters, with data expected in the fall.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education.
Copyright 2021 Associated Press. All rights reserved.
Source: https://apnews.com/article/coronavirus-vaccine-pfizer-approval-1361ff61d06b815652a08a7cc0683a72