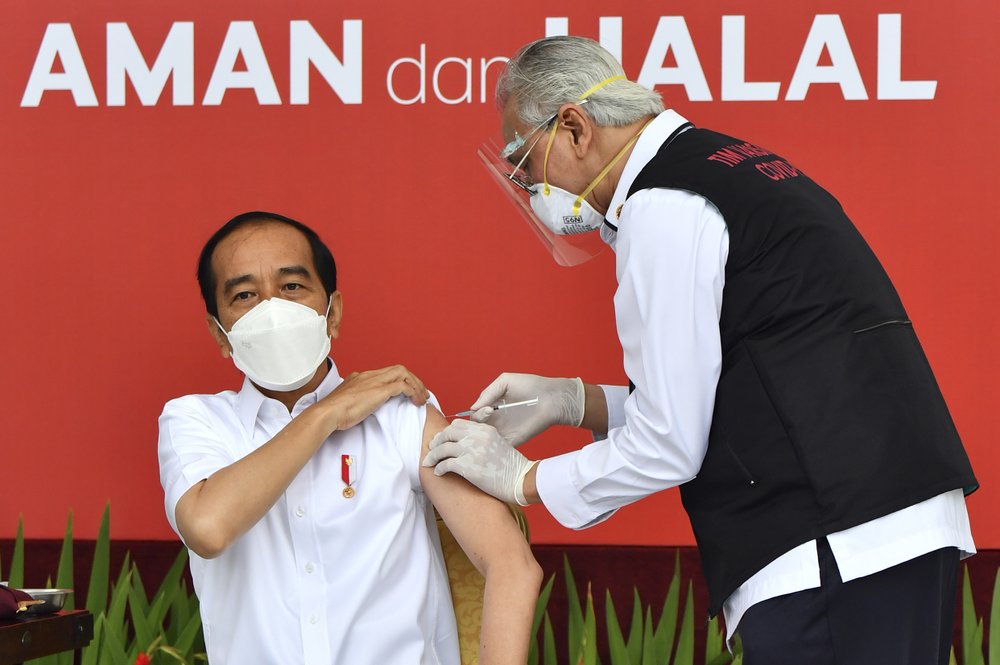
In this photo released by Indonesian Presidential Palace, President Joko Widodo, left, receives a shot of COVID-19 vaccine at Merdeka Palace in Jakarta, Indonesia, Wednesday, Jan. 13, 2021. Widodo on Wednesday received the first shot of a Chinese-made COVID-19 vaccine after Indonesia approved it for emergency use and began efforts to vaccine millions of people in the world’s fourth most populated country. Writings on the banner in the background read “Safe and Halal.” (Agus Suparto/Indonesian Presidential Palace via AP)
JAKARTA, Indonesia (AP) — Indonesian President Joko Widodo received the first shot of a Chinese-made coronavirus vaccine Wednesday after the government authorized it for emergency use and began efforts to vaccinate millions of people across the vast archipelago in one of the world’s most populous countries.
Indonesia’s vaccination program is the first large-scale use outside of China of the Sinovac Biotech Ltd. vaccine. It poses massive challenges in a country whose thousands of islands stretch across an area about as wide as the continental United States and where transportation and infrastructure are limited in many places. Health officials have also noted it will be difficult to keep the vaccine at the required 36–46 degrees Fahrenheit (about 2-8 degrees Celsius) to maintain its safety and effectiveness.
After President Widodo, top military, police and medical officials also received shots, as did the secretary of the Indonesian Ulema Council, the clerical body that last week ruled the vaccine was halal, or acceptable for use under Islamic law.
A health care worker, businesspeople and a social media influencer also got the vaccine to encourage others to follow suit once it is available to them. Officials have said they will prioritize health care workers, civil servants and other at-risk populations, and the two-dose vaccine will be free for all Indonesian citizens.
“We need to do the vaccination to stop the chain spread of COVID-19 and give health protection to us and the safety to all Indonesian people. It will also help accelerate economic improvement,” Widodo said.
While the Pfizer-BioNTech vaccine has been greeted with much fanfare in the West, its relatively high price and requirement for ultra-cold storage mean that other shots, like Chinese, Russian and the AstraZeneca vaccines, are more likely to be distributed to much of the developing world, even as experts have said more data needs to be shared about the Chinese and Russian products.
Indonesia plans to vaccinate two-thirds of its population of about 270 million people — or just over 180 million people. That means it needs about 427 million shots, given the estimate that 15% may be wasted, Health Minister Budi Gunadi Sadikin said.
“This vaccine is the instrument we can use to protect us. But more importantly, the vaccine is the instrument to protect our family, our neighbor, Indonesian people and the human civilization,” Sadikin said on Wednesday.
He noted that given Indonesia’s enormous population — the world’s fourth largest — its vaccination program is key to worldwide efforts to protect enough people so that the global community reaches herd immunity.
But he cautioned that great obstacles remain.
“We know that the cold-chain distribution is not complete. This is the obstacle,” Sadikin said this week. “We are worried.”
The rollout comes as Indonesia registered the daily record in COVID-19 infections and fatalities on Wednesday, with 11,278 new cases and 306 new deaths reported in the last 24 hours. The country has recorded more than 858,000 infections and over 24,900 deaths.
Some scientists warn that not enough data has been published about the effectiveness or safety of the Sinovac vaccine. It has yet to be tested in tens of thousands of people in the kind of rigorous study considered necessary before being licensed for wide use.
But Zullies Ikawati, a pharmacy expert from Gadjah Mada University, said the efficacy rate shown so far from Indonesia’s small late-stage trials is good start and could save the lives of many people.
“Of course we still have to wait for the effectiveness of the vaccine after it is used in the community,” Ikawati said. “Observations on the efficacy and safety will still be carried out for the next six months to get full approval.”
Besides Indonesia, the Sinovac vaccine has been granted conditional use authorization in China and Bolivia. Several other countries have purchase agreements for millions of doses, including the Philippines, Singapore and Ukraine.
Chinese health officials have said that some 9 million vaccine doses have been administered in China, though the number of people who received the Sinovac shot itself has not been disclosed. China has several vaccines in development.
Indonesia received its first shipment of the Sinovac vaccines on Dec. 6 and began distributing the doses around the country while awaiting emergency use authorization. It was cleared for that use based on clinical trial data and after the Indonesian Ulema Council declared the vaccine halal.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education. The AP is solely responsible for all content.
Copyright 2020 Associated Press. All rights reserved.